Based on molecular imaging technology, services scope:
• Clinical study protocol design and drafting;
• Selecting sites to be involved;
• Communication with Ethic committee;
• Sites' monitoring;
• Data management & Statistical analysis;
• Clinical study report design and drafting
A bridging study is defined as a study performed in the new region to provide pharmacodynamic or clinical data on efficacy, safety, dosage and dose regimen in the new region that will allow extrapolation of the foreign clinical data to the population in the new region. A bridging study for efficacy could provide additional pharmacokinetic information in the population of the new region. All regions acknowledge the desirability of utilizing foreign clinical data that meet the regulatory standards and clinical trial practices acceptable to the region considering the application for registration.
However, concern that ethnic differences may affect the medication's safety, efficacy, dosage and dose regimen in the new region has limited the willingness to rely on foreign clinical data. Historically, this has been one of the reasons, therefore, the regulatory authority in the new region has often requested that all, or much of, the foreign clinical data in support of registration be duplicated in the new region. Although ethnic differences among populations may cause differences in a medicine’s safety, efficacy, dosage or dose regimen, many medicines have comparable characteristics and effects across regions. Requirements for extensive duplication of clinical evaluation for every compound can delay the availability of new therapies and unnecessarily waste drug development resources.
Classification of instrinsic and extrinsic ethic factors:
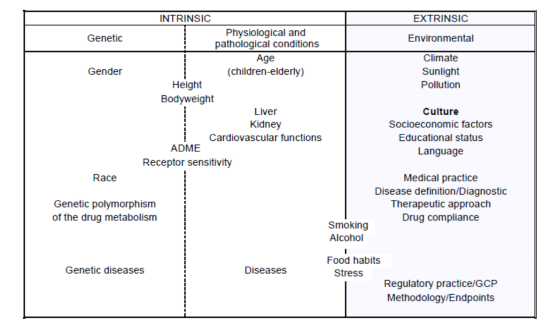
MITRO uses PET/SPECT and other molecular imaging technologies to provide ethnic difference research services for new drugs. With these services, PK/PD data can be used to evaluate drug sensitivity to ethnic factors, and bridging studies can be performed to provide clinical information on PD and/or efficacy, dose and dosage regime for a new area, so that overseas clinical data can be used in people in a different region.